較為安全的離子液體型發動機燃料——多氰基離子液體
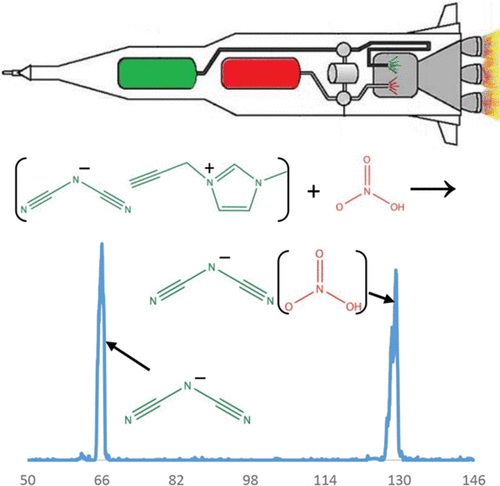
Dicyanamide [N(CN)2–] is a common anionic component of ionic liquids, several of which have shown hypergolic reactivity upon mixing with white-fuming nitric acid. In this study, we explore the thermochemistry of dicyanamide and its reactivity with nitric acid and other molecules to gain insight into the initial stages of the hypergolic phenomenon. We have developed and utilized an electrospray ion source for our selected ion flow tube (SIFT) to generate the dicyanamide anion. We have explored the general reactivity of this ion with several neutral molecules and atoms. Dicyanamide does not show reactivity with O2, H2SO4, H2O2, DBr, HCl, NH3, N2O, SO2, COS, CO2, CH3OH, H2O, CH4, N2, CF4, or SF6 (k < 1 × 10–12 cm3/s); moreover, dicyanamide does not react with N atom, O atom, or electronically excited molecular oxygen (k < 5 × 10–12 cm3/s), and our previous studies showed no reactivity with H atom. However, at 0.45 Torr helium, we observe the adduct of dicyanamide with nitric acid with an effective bimolecular rate constant of 2.7 × 10–10 cm3/s. Intrinsically, dicyanamide is a very stable anion in the gas phase, as illustrated by its lack of reactivity, high electron-binding energy, and low proton affinity. The lack of reactivity of dicyanamide with H2SO4 gives an upper limit for the gas-phase deprotonation enthalpy of the parent compound (HNCNCN; <310 ± 3 kcal/mol). This limit is in agreement with theoretical calculations at the MP2/6-311++G(d,p) level of theory, finding that ΔH298?K(HNCNCN) = 308.5 kcal/mol. Dicyanamide has two different proton acceptor sites. Experimental and computational results indicate that it is lower in energy to protonate the terminal nitrile nitrogen than the central nitrogen. Although proton transfer to dicyanamide was not observed for any of the acidic molecules investigated here, the calculations on dicyanamide with one to three nitric acid molecules reveal that higher-order solvation can favor exothermic proton transfer. Furthermore, the formation of 1,5-dinitrobiuret, proposed to be the key intermediate during the hypergolic ignition of dicyanamide ionic liquids with nitric acid, is investigated by calculation of the reaction coordinate. Our results suggest that solvation dynamics of dicyanamide with nitric acid play an important role in hypergolic ignition and the interactions at the droplet/condensed-phase surface between the two hypergolic liquids are very important. Moreover, dicyanamide exists in the atmosphere of Saturn’s moon, Titan; the intrinsic stability of dicyanamide strongly suggests that it may exist in molecular clouds of the interstellar medium, especially in regions where other stable carbon–nitrogen anions have been detected.